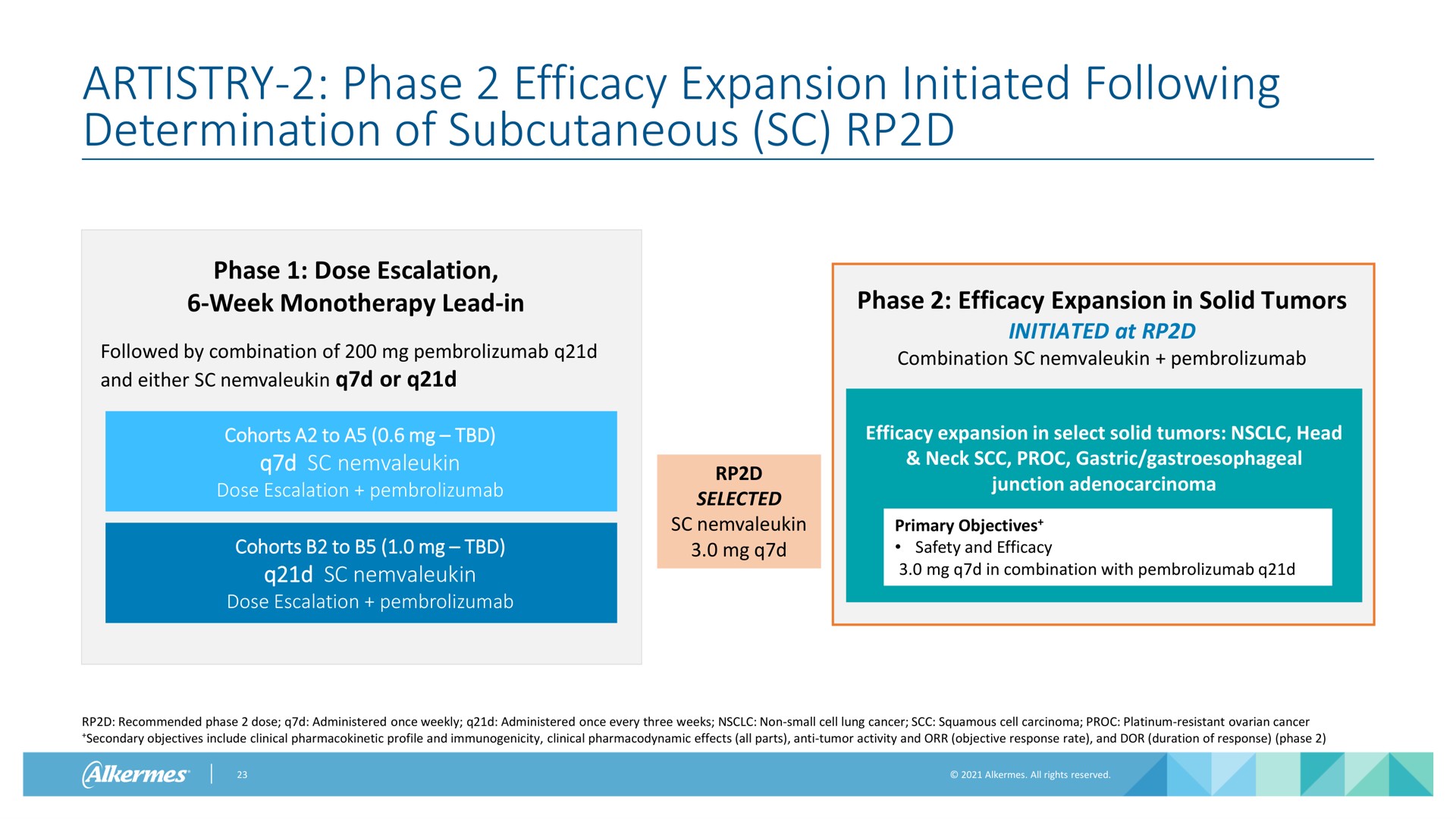
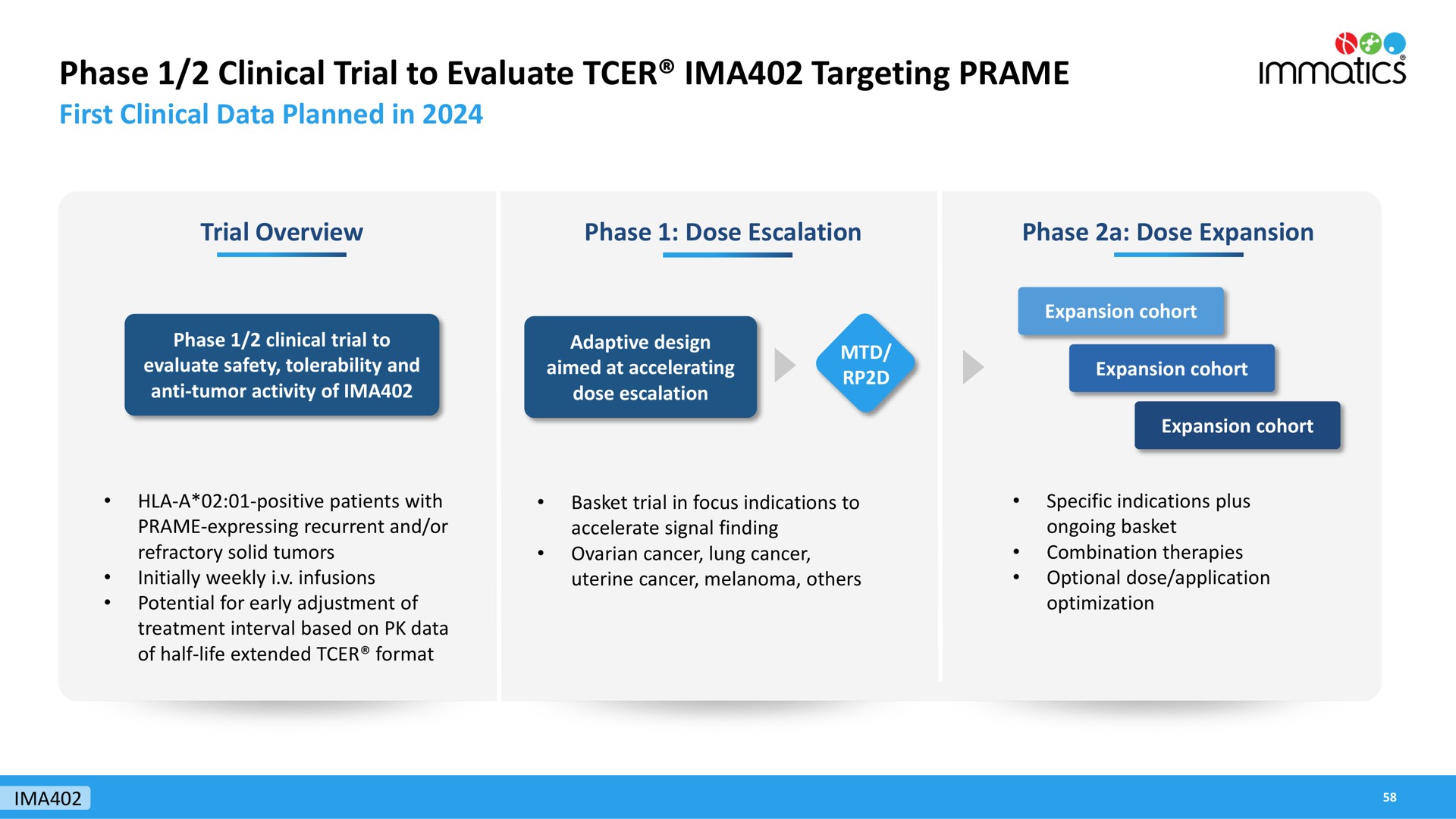
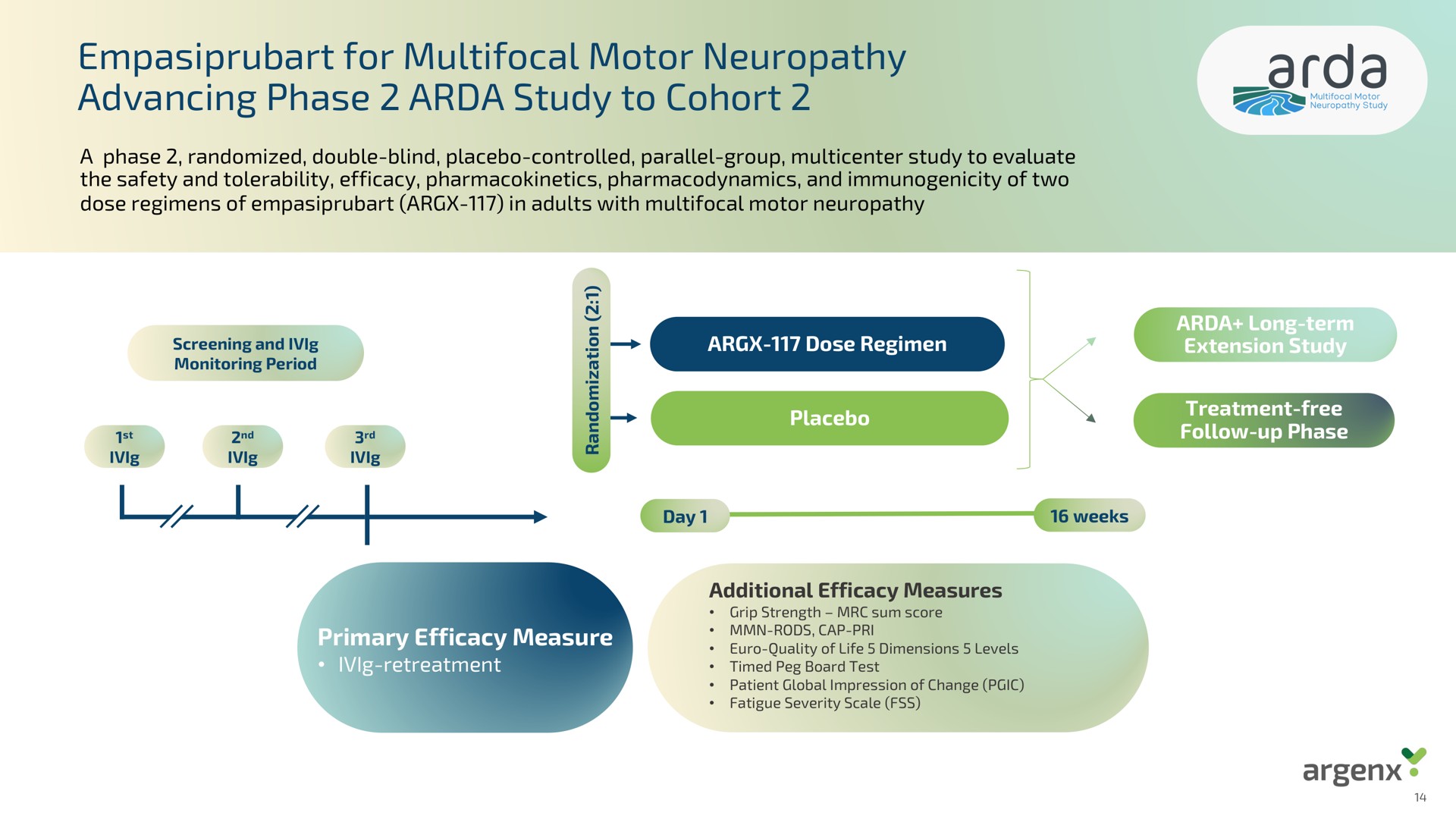
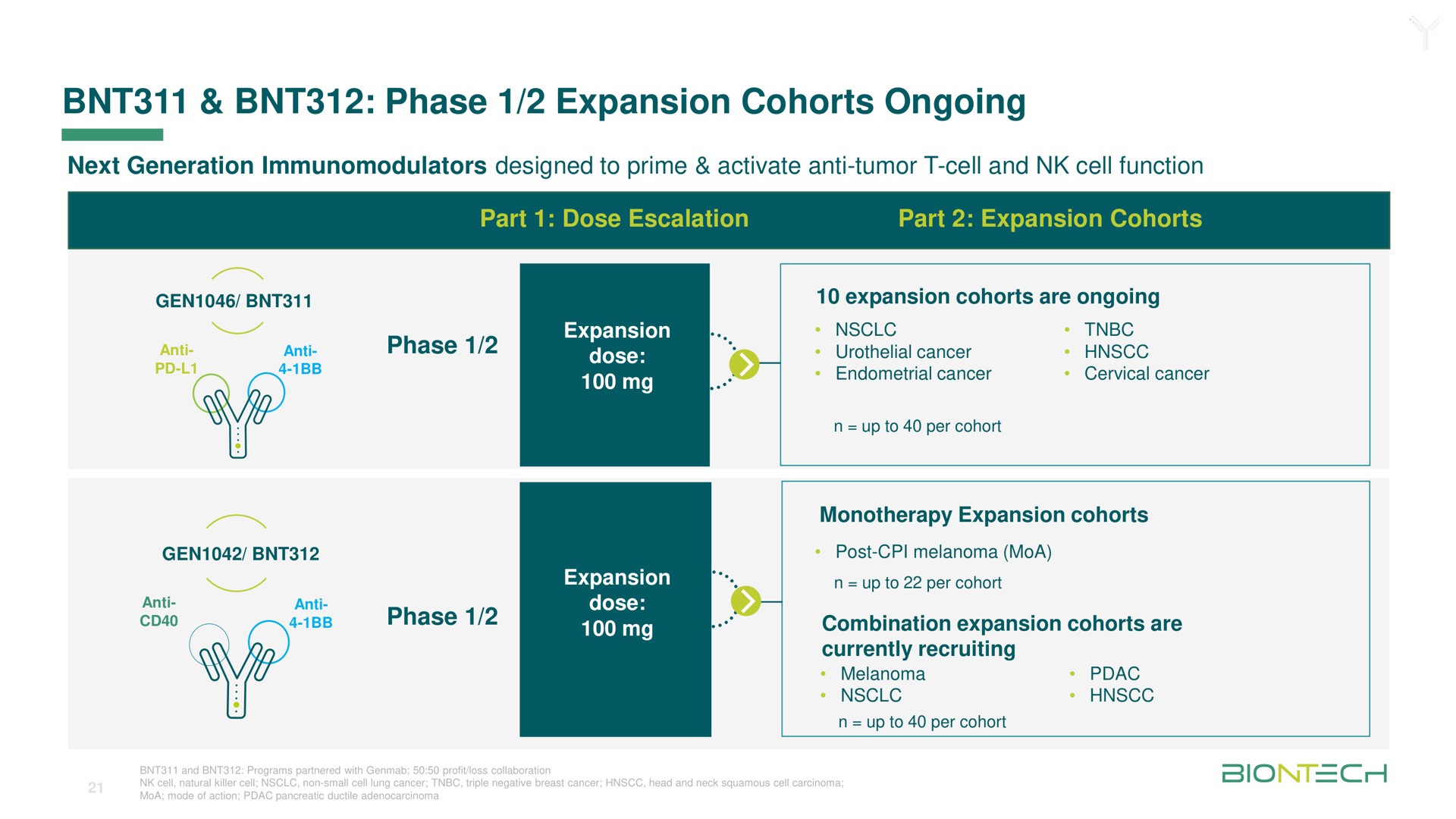
Alkermes
Company
Deck Type
Sector
Deck date
March 2021
Slide
80 of 144
Related slides by other companies
Other recent decks by Alkermes
Search Thousands of Presentations by World Leading Companies
Stay in the loop
Join our mailing list to stay in the loop with updates and newest feature releases

© 2021-2023 Slidebook.io