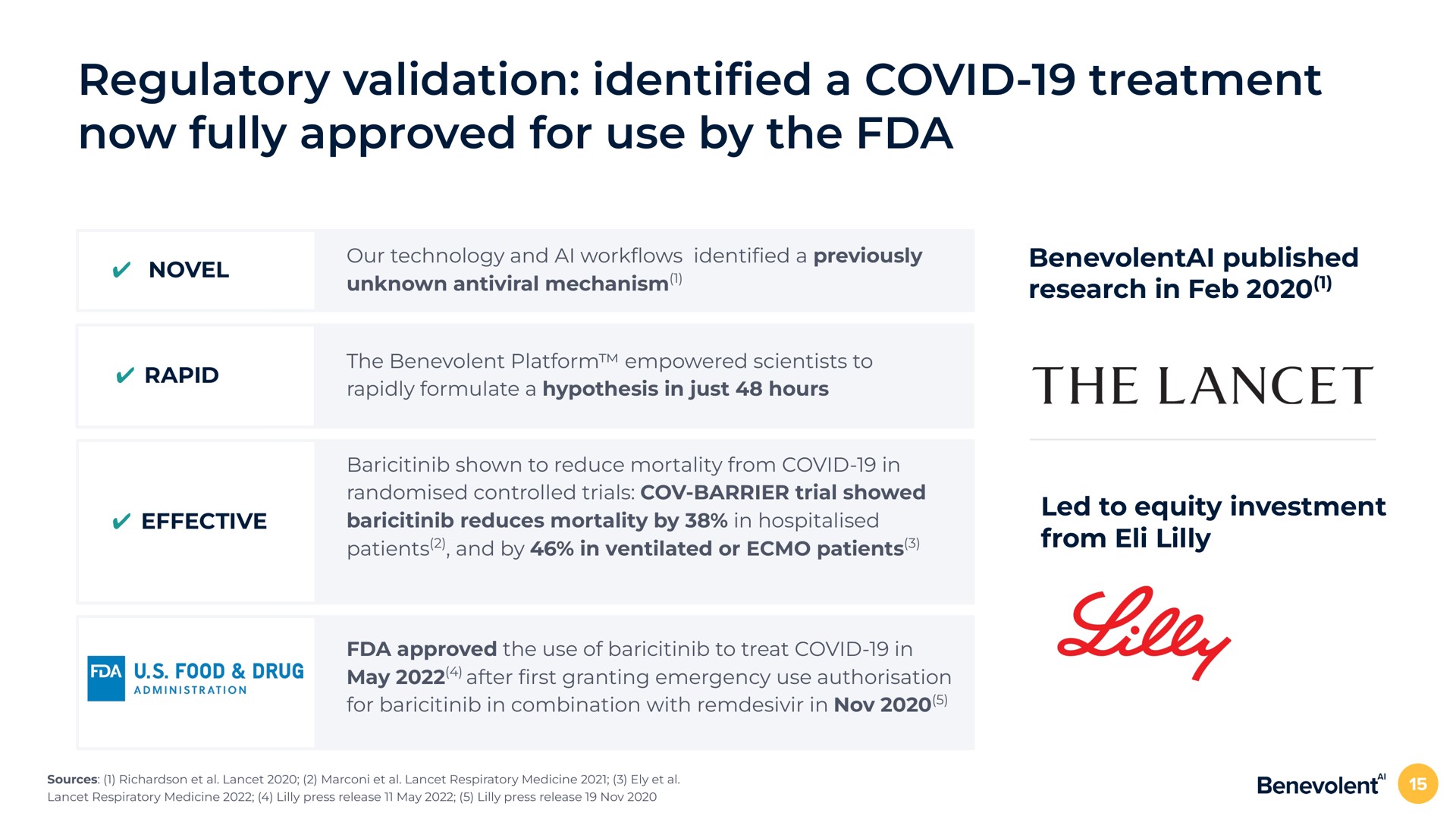
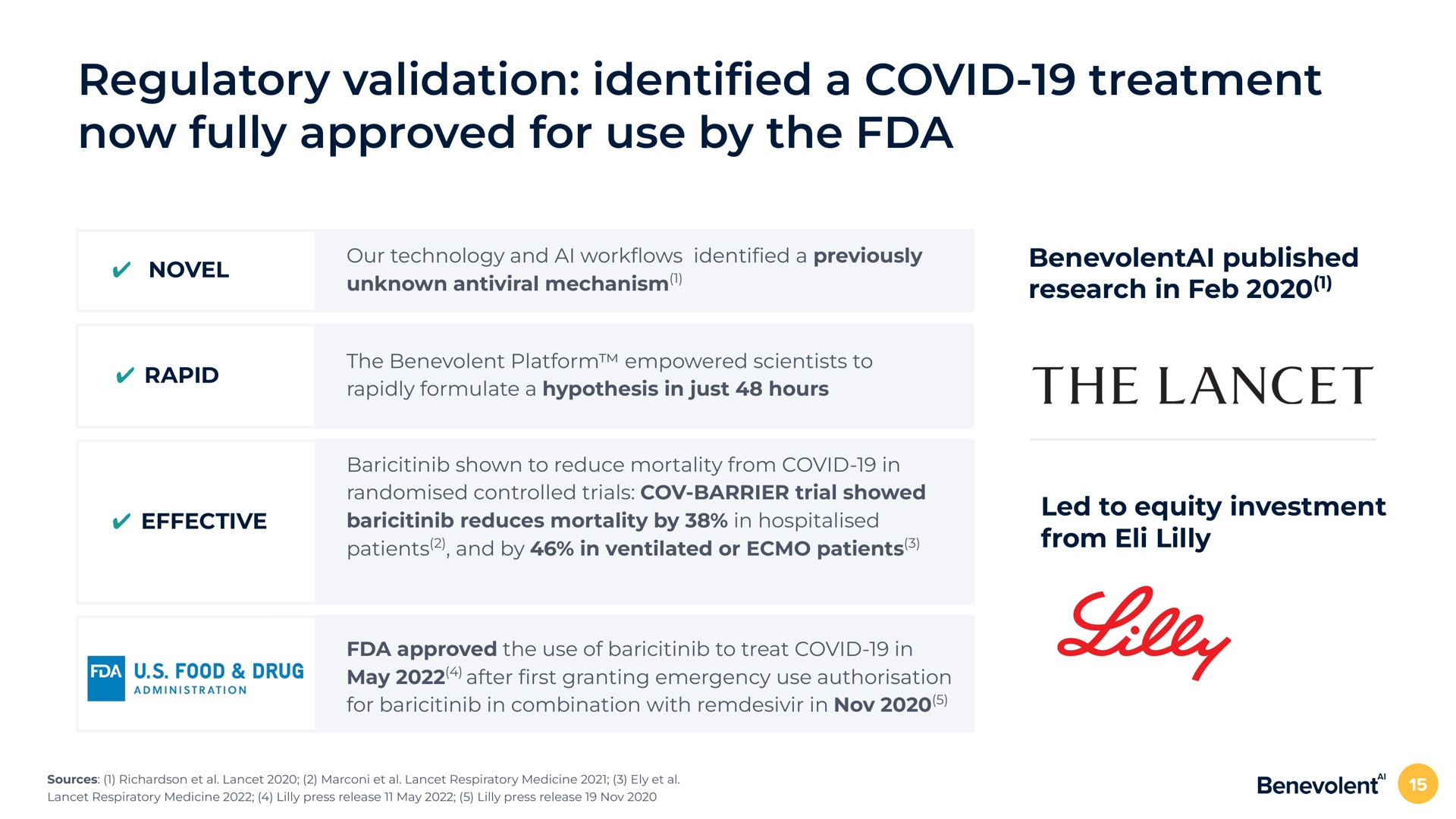
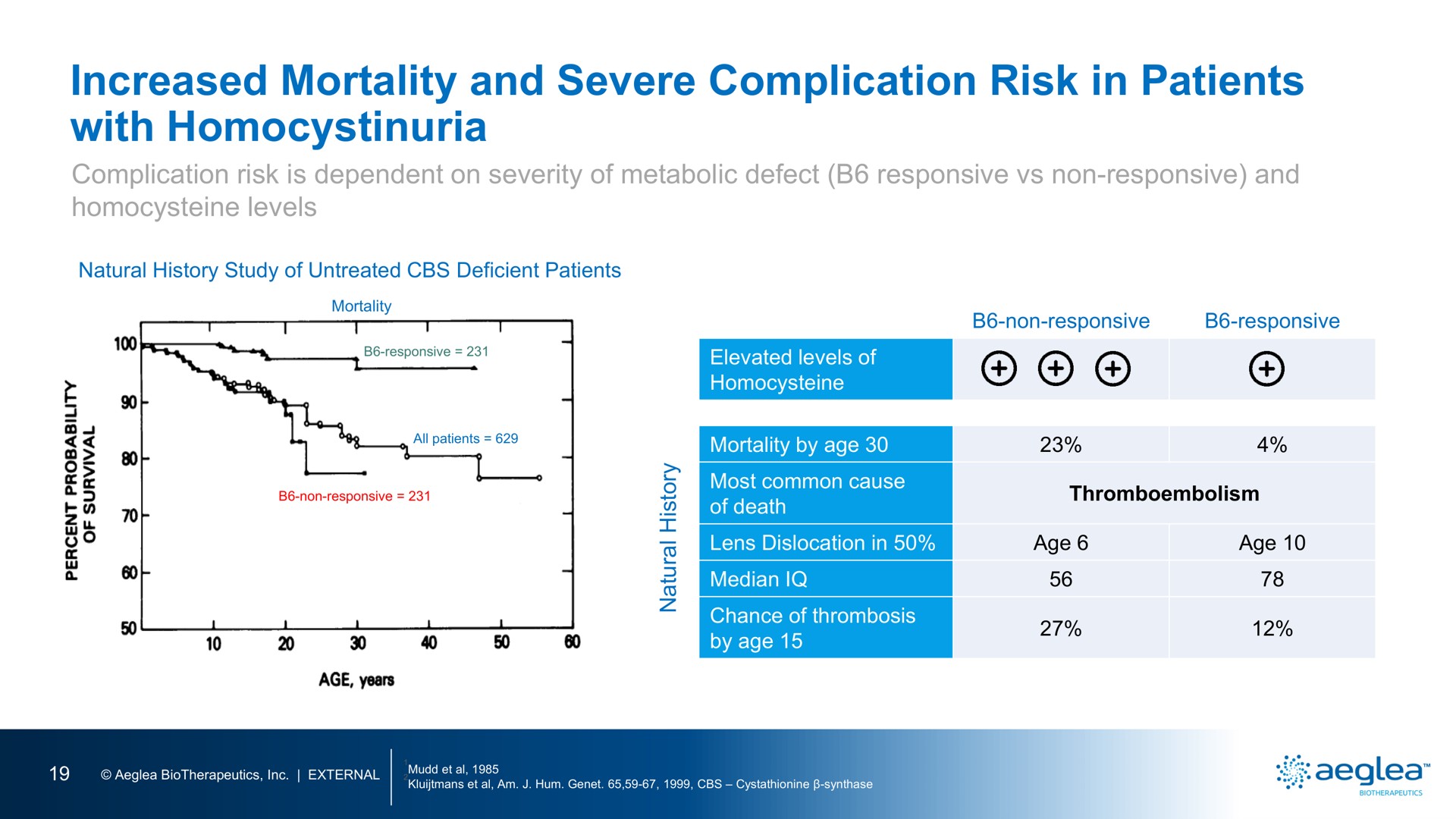
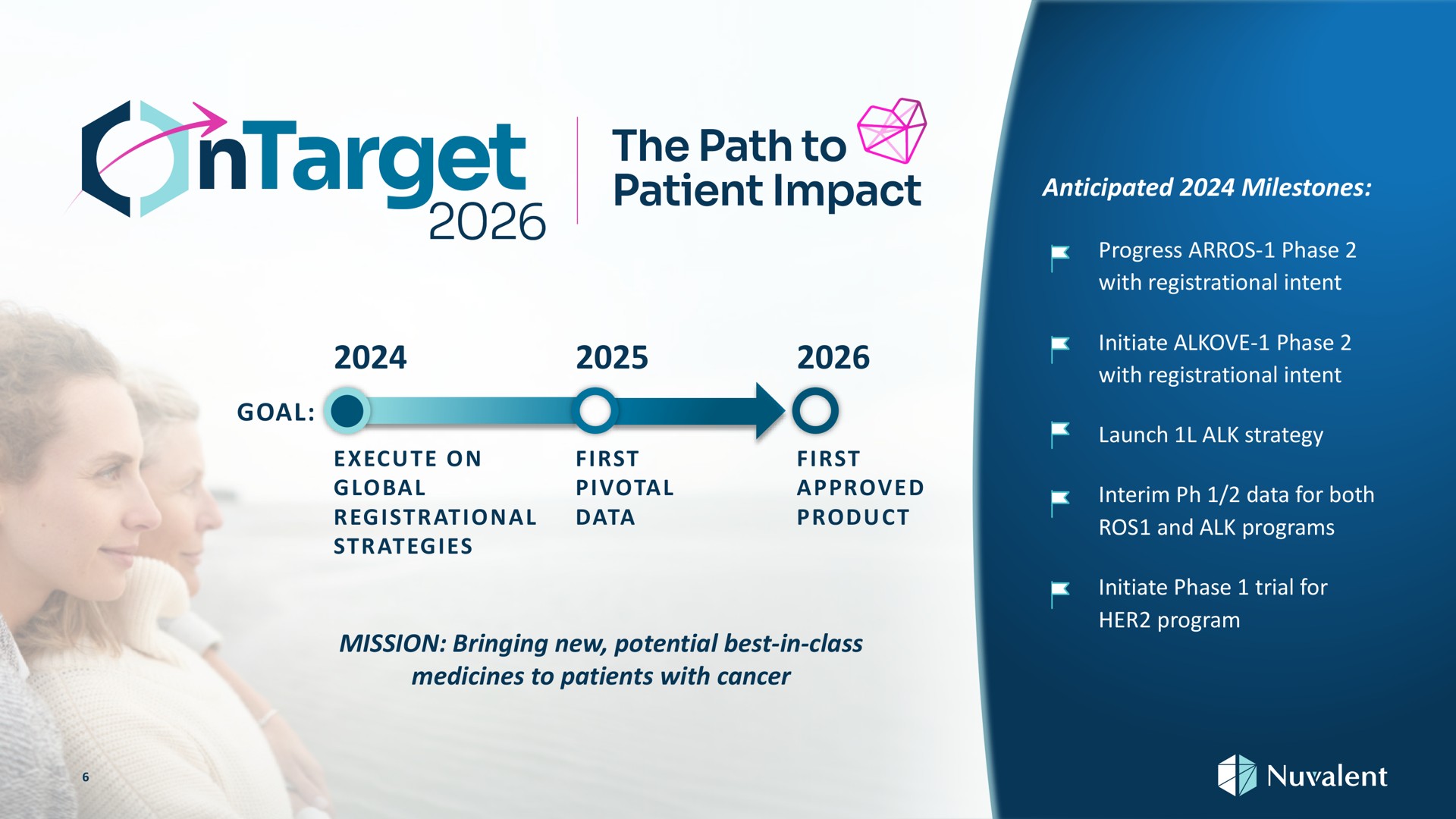
BenevolentAI
Company
Deck Type
Sector
Industry
Deck date
September 2022
Slide
25 of 74
Similar slides by BenevolentAI
Related slides by other companies
Investor Presentation
August 2020
Investor Presentation
January 2024
Investor Presentation
January 2024
Investor Presentation
January 2024
Other recent decks by BenevolentAI
Search Thousands of Presentations by World Leading Companies
Stay in the loop
Join our mailing list to stay in the loop with updates and newest feature releases

© 2021-2023 Slidebook.io